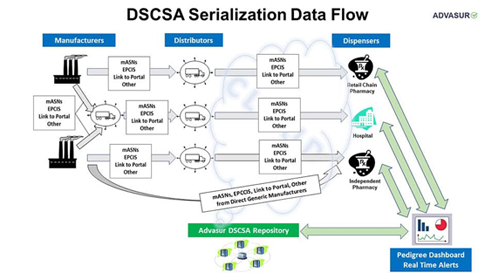
DSCSA (also known as Track and Trace) is a non-funded government mandate that is rolling out in three phases that began in 2013 and is on schedule to be fully mandated by 11/27/2023. Advasur 360 Compliance Services is a comprehensive suite of Drug Supply Chain Security Act (DSCSA) services that will help you with all current and future DSCSA requirements.
FDA COMPLIANCE — TRACK AND TRACE
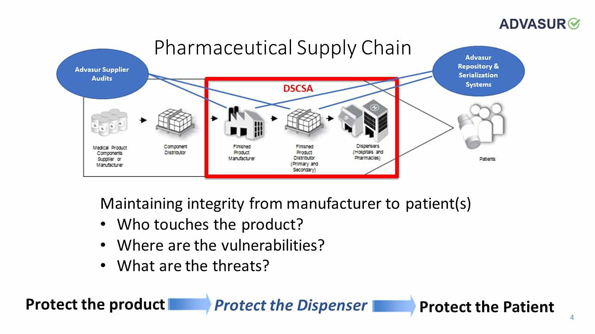
DSCSA Compliance

Goals of the Drug Quality and Security Act
The System will:
- Facilitate the exchange of information by trading partners at the individual package level
- Improve efficiency of recalls
- Enable prompt response to suspect and illegitimate products when found
- Create transparency and accountability in the drug supply chain
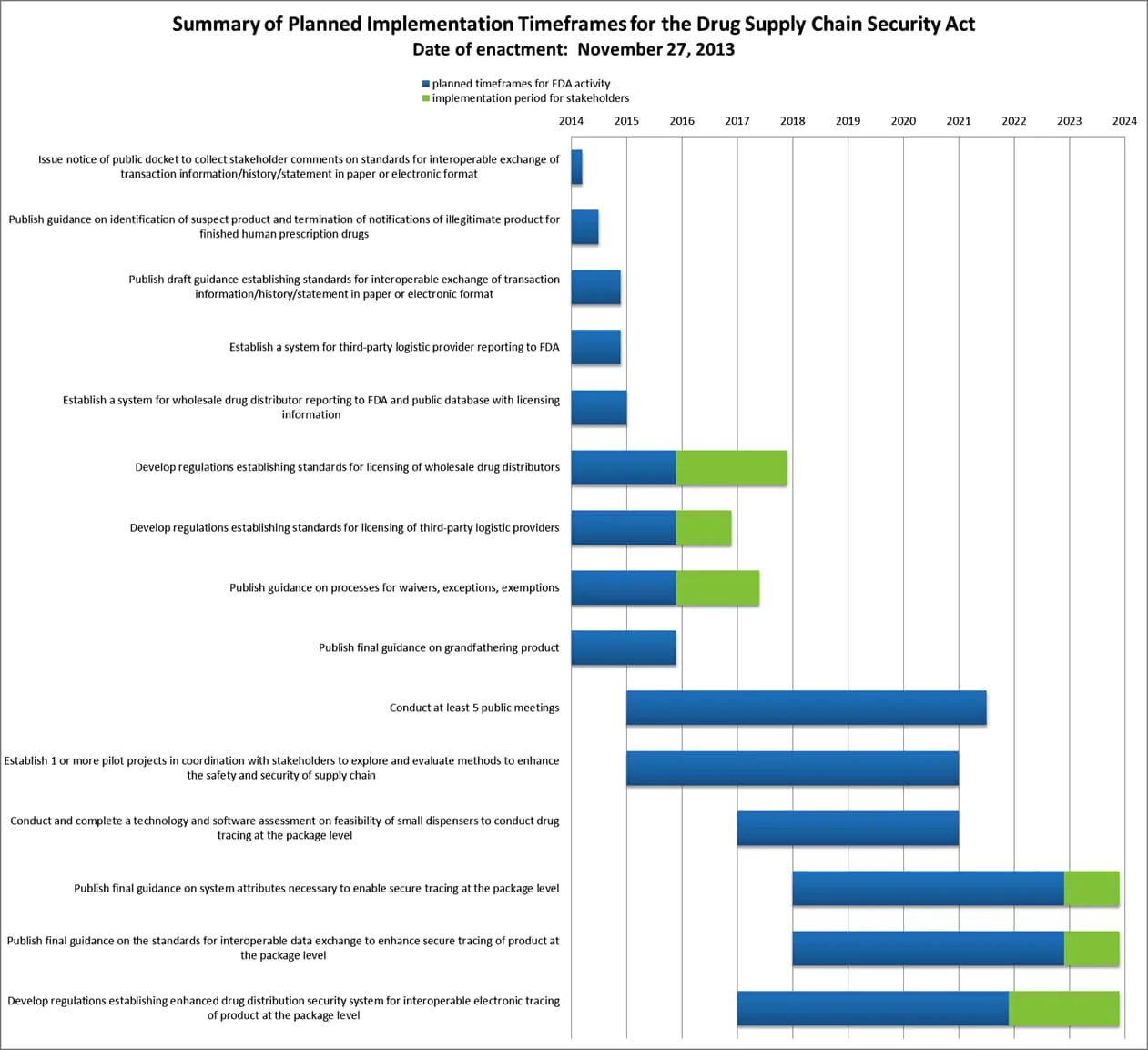
Summary of the Planned Implementation Timeframes for the DSCSA
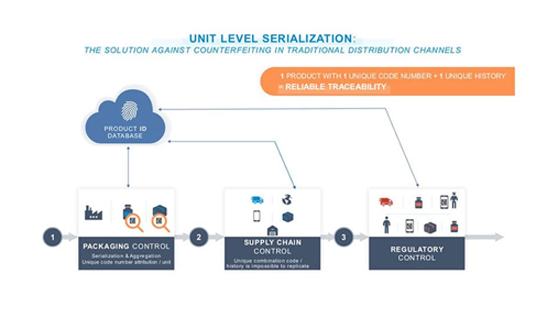
What Is Serialization
and What Does It Mean to the Pharma Industry?
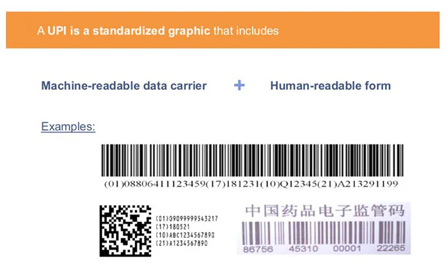
In the U.S., the Unique Product Identifier (UPI) consists of:
A National Drug Code (NDC) or a Global Trade Item Number (GTIN)
A Serial Number (alphanumeric up to 20 characters)
A Lot Number
An Expiration Date
Serialization Challenges For Dispensers
Timeliness: If you cannot achieve serialization compliance in time, you put your whole business at risk Productivity: Central configuration and prequalification reduce impact on OEE and speed up deployment Connectivity: Serial numbers, master data and event information need to be exchanged among supply chain parties and also reported in a compliant way. Communication: Serialization implementation requires the coordination of numerous internal and external stakeholders. Don’t underestimate the scope of your project! Serialization and Beyond: when choosing your serialization solution, don’t forget to consider future compliance and regulatory requirements. Beyond compliance, think of the business potential of your serialization data.